Doxepinum
Appearance
 |
Cave: notitiae huius paginae nec praescriptiones nec consilia medica sunt. |
| Cognitores | |
|---|---|
| ChemSpider | 3046 |
| PubChem | 3158 |
| DrugBank | DB01142 |
| Natura chemica | |
| |
| |
| Formula chemica | C 19H 21NO |
| Massa molaris | 279.376 g/mol |
| Natura pharmacologica | |
| Codex ATC | N06AA12 (WHO) |
| Tempus semivitae biologicum | 17 (8-24) h Nor-Doxepinum: 31 h |
| Metabolismus | Iecore (hepaticus): CYP2D6 CYP2C19 |
| Metabolitus | Nor-Doxepinum |
| Excretio | renibus (ca. 50%), faecibus: minus |
| Ad usum therapeuticum | |
| Applicatio | per os, i.v., i.m. |
| MedlinePlus | a682390 (Anglice) |
Doxepinum est substantia psychoanaleptica et antidepressiva, ergo medicamentum tractationis depressionum, praesertim cum insomnia praesente, effectu sedativo suo causa, quia Doxepinum receptorium H1 agonista inversa[1] obsidet. Per structuram chemicam Doxepinum est antidepressivum tricyclicum. Ceroma[2] quoque Doxepinum therapiae pruritus[3] et dermatitis atopicae et lichenis simplicis chronici (neurodermatitis) brevis temporis adhiberi potest.
Natura Doxepini
[recensere | fontem recensere]Natura chemica
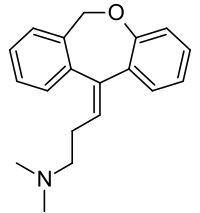
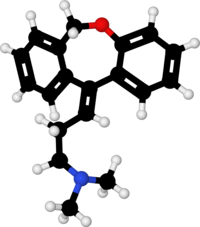
[recensere | fontem recensere]Formula summarum est C
19H
21NO. Nomen IUPAC est 3-(dibenzo[b,e]oxepino-11(6H)-ylideno)-N,N-dimethylo-propano-1-aminum.
Massa molaris est 279.376 g/mol.
Nexus interni
Notae
[recensere | fontem recensere]- ↑ Shiroishi M, Kobayashi T (2017). "Structural Analysis of the Histamine H1 Receptor". Handb Exp Pharmacol 241: 21-30
- ↑ Leppert W, Malec-Milewska M, Zajaczkowska R, Wordliczek J (2018). "Transdermal and Topical Drug Administration in the Treatment of Pain". Molecules 23 (3): pii:E681
- ↑ Kaur R, Sinha VR (2018). "Antidepressants as antipruritic agents: A review". Eur Neuropsychopharmacol 28 (3): 341-52
